SAMINOR 2
The second survey of the Population-based Study on Health and Living Conditions in Regions with Sami and Norwegian Populations (SAMINOR 2) was conducted by the Centre for Sami Health Research at the Department of Community Medicine, UiT The Arctic University of Norway in 2012-2014.
SAMINOR 2 was conducted in two stages. Stage 1 was a questionnaire survey conducted in 25 municipalities with Sami and Norwegian populations in Northern Norway and Trøndelag. The survey took place in the same regions as SAMINOR 1, but Sør-Varanger was included as a new municipality. In six of the municipalities, only a few districts were included. Stage 2 (the clinical survey) was conducted in ten selected municipalities that had all taken part in SAMINOR 1 and SAMINOR 2, Stage 1.
SAMINOR 2, Stage 1: The Questionnaire Survey
In January 2012, an eight-page questionnaire was sent to everyone aged 18-69 in the selected region, accompanied by an invitation letter (invitation letter in Norwegian and Northern Sami, invitation letter in Norwegian and Lule Sami, invitation letter in Norwegian and Southern Sami). The questionnaire was provided in Northern Sami, Lule Sami, and Southern Sami, in addition to Norwegian. The questionnaire could also be completed online (Norwegian only). The Centre for Sami Health Research used Statistics Norway to send out the questionnaires. Reminders (reminder in Norwegian and Northern Sami, reminder in Norwegian and Lule Sami, reminder in Norwegian and Southern Sami) were sent twice to non-responders at about six weeks and four months after the original invitation.
Municipalities invited to participate in stage 1:
Finnmark:
Karasjok, Kautokeino, Porsanger, Tana, Nesseby, Lebesby, Alta, Loppa. Kvalsund and Sør-Varanger.
Troms:
Kåfjord, Kvænangen, Storfjord, Lyngen, Skånland and Lavangen
Nordland:
Tysfjord, Evenes, Hattfjelldal (Hattfjelldal district), Grane (Majavatn district), Narvik (Vassdalen district)
Nord-Trøndelag:
Røyrvik and parts of Namsskogan (Trones and Furuly) and Snåsa (Vinje)
Sør-Trøndelag:
Parts of Røros Municipality (Brekken)
Apart from Alta and Sør-Varanger, all municipalities had fewer than 4000 inhabitants.
Participation in Stage 1 (the SAMINOR 2 Questionnaire Survey)
|
|
Men |
Women |
Total |
||||||
|
Age |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
|
18-19 |
966 |
100 |
10.4 |
844 |
173 |
20.5 |
1 810 |
273 |
15.1 |
|
20-29 |
3 987 |
426 |
10.7 |
3 610 |
785 |
21.7 |
7 597 |
1 211 |
15.9 |
|
30-39 |
3 778 |
680 |
18.0 |
3 586 |
965 |
26.9 |
7 364 |
1 645 |
22.3 |
|
40-49 |
4 876 |
1 096 |
22.5 |
4 586 |
1 548 |
33.8 |
9 462 |
2 644 |
27.9 |
|
50-59 |
4 592 |
1 339 |
29.2 |
4 236 |
1 594 |
37.6 |
8 828 |
2 933 |
33.2 |
|
60-69 |
4 366 |
1 508 |
34.5 |
3 818 |
1 386 |
36.3 |
8 184 |
2 894 |
35.4 |
|
Total |
22 565 |
5 149 |
22.8 |
20 680 |
6 451 |
31.2 |
43 245 |
11 600 |
26.8 |
For further details of SAMINOR 2 Stage 1 (the Questionnaire Survey), see this article:
- Invitation letter, Norwegian and Northern Sami
- Invitation letter, Norwegian and Lule Sami
- Invitation letter, Norwegian and Southern Sami
- Questionnaire, Norwegian
- Questionnaire, Northern Sami
- Questionnaire, Lule Sami
- Questionnaire, Southern Sami
- Questionnaire, English translation
- Reminder letter, Norwegian and Northern Sami
- Reminder letter, Norwegian and Lule Sami
- Reminder letter, Norwegian and Southern Sami
SAMINOR 2, Stage 2: The SAMINOR 2 Clinical Survey
The SAMINOR 2 Clinical Survey, also called the “Health and Lifestyle Survey”, was conducted by the Centre for Sami Health Research in 2012-2014 in ten selected municipalities, all of which participated in SAMINOR 1 and in Stage 1 of SAMINOR 2. This survey included a new questionnaire, some clinical examinations and blood sampling.
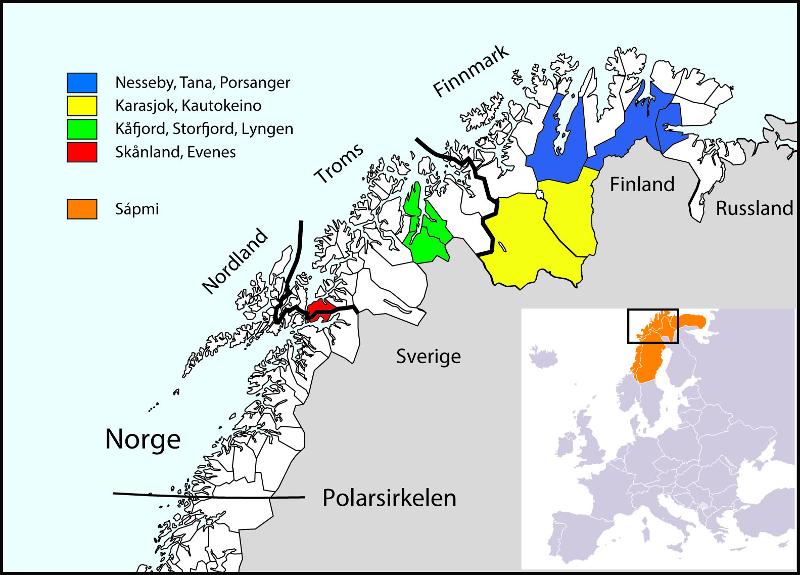
Municipalities selected for participation in SAMINOR 2, Stage 2
- Skånland and Evenes (September-October 2012)
- Karasjok (January-February 2013)
- Kautokeino (February-March 2013)
- Porsanger (April-May 2013)
- Kåfjord (September-October 2013)
- Storfjord (October-November 2013)
- Nesseby and Tana (February-April 2014)
- Lyngen (May-June 2014).
Implementation of the survey
Prior to the survey, cooperation was established with the authorities in each municipality. These provided suitable premises, freed municipal health workers to work in the clinical survey and offered other assistance. The Centre for Sami Health Research rented or borrowed premises in the various municipalities and established a research station for the period of the survey. Mostly, local health workers were freed to work at the research station. Seventy hired staff were involved in collecting the data.
The target group was the entire population aged 40–79, regardless of whether or not they participated in Stage 1. About one month in advance, a pamphlet was sent out by post, which contained information about the forthcoming health survey (pamphlet in Northern Sami, pamphlet in Kven). A few weeks later, a personal invitation was sent out, which included an information brochure (information brochure in Northern Sami), a scheduled appointment (appointment in Northern Sami) and a questionnaire. About halfway through the survey period, those who had not yet attended received a reminder in the post (reminder in Northern Sami).
The survey consisted of a clinical examination and blood tests. Participants were also asked to complete in advance a questionnaire they had been sent and bring it to the appointment. Participants aged 70–79 received a separate questionnaire designed for older people. There were thus two questionnaires used in Stage 2: one of eight pages for people aged 40–69 years (questionnaire in Norwegian, questionnaire in Northern Sami) and one of four pages for the age group 70–79 years (questionnaire in Norwegian, questionnaire in Northern Sami).
Clinical measurements
Height, weight, hip and waist circumference, pulse and blood pressure were measured.
Blood samples
Non-fasting blood samples were taken. Long-term blood sugar (HbA1c) and haemoglobin levels were analysed on site. All participants were offered written results of the examinations at the station, including long-term blood sugar and haemoglobin levels. In the case of abnormal findings, participants were referred to their GP. Other samples were frozen and sent to UiT The Arctic University of Norway to be stored in the biobank. Lipids, biomarkers for diabetes, inflammatory reactions, iron status and some vitamins were analysed at the University Hospital of North Norway in autumn 2014. In spring 2016, vitamin D was analysed in Helsinki, Finland. Environmental toxins were also analysed in selected participants. The remaining blood samples were stored in the biobank for later analysis.
Participation in Stage 2 (the SAMINOR 2 Clinical Survey)
|
|
Men |
Women |
Total |
||||||
|
Age |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
|
40-44 |
867 |
255 |
29.4 |
836 |
388 |
46.4 |
1 703 |
643 |
37.8 |
|
45-49 |
907 |
283 |
31.2 |
795 |
364 |
45.8 |
1 702 |
647 |
38.0 |
|
50-54 |
883 |
319 |
36.1 |
777 |
406 |
52.3 |
1 660 |
725 |
43.7 |
|
55-59 |
897 |
372 |
41.5 |
848 |
481 |
56.7 |
1 745 |
853 |
48.9 |
|
60-64 |
970 |
481 |
49.6 |
872 |
535 |
61.4 |
1 842 |
1 016 |
55.2 |
|
65-69 |
930 |
488 |
52.5 |
817 |
504 |
61.7 |
1 747 |
992 |
56.8 |
|
70-74 |
591 |
336 |
56.9 |
550 |
333 |
60.5 |
1 141 |
669 |
58.6 |
|
75-79 |
424 |
213 |
50.2 |
491 |
246 |
50.1 |
915 |
459 |
50.2 |
|
Total |
6 469 |
2 747 |
42.5 |
5 986 |
3 257 |
54.4 |
12 455 |
6 004 |
48.2 |
For further details of SAMINOR 2 Stage 2 (the Clinical Survey), see this article:
- Pamphlet sent out 3-4 weeks prior to start, Norwegian
- Pamphlet, Northern Sami
- Pamphlet, Porsanger, Kven
- Pamphlet, Storfjord, Kven
- Information brochure, Norwegian
- Information brochure, Northern Sami
- Information brochure, Porsanger, Kven
- Information brochure, Storfjord, Kven
- Invitation letter, Skånland/Evenes, Norwegian
- Invitation letter, Karasjok, Northern Sami
- Questionnaire 40-69 years, eight pages, Norwegian
- Questionnaire 40-69 years, eight pages, Northern Sami
- Questionnaire 40-69 years, eight pages, English translation
- Questionnaire 70-79 years, four pages, Norwegian
- Questionnaire 70-79 years, four pages, Northern Sami
- Questionnaire 70-79 years, four pages, English translation
- Clinical measurement form
- Lab form
- Declaration of consent, Norwegian
- Declaration of consent, Northern Sami
For researchers:
- Questionnaire 40-69 years, including names of variables and coding
- Questionnaire 70-79 years, including names of variables and coding
Feedback to municipalities
It is important to return knowledge to the people being researched. With this in mind, reports were prepared for all municipalities included in the SAMINOR 2 Clinical Survey with selected results from the municipality concerned. These results can contribute to health prevention and promotion and improve social services in the north. The SAMINOR Study can therefore be a useful contribution to meeting the requirements of the the Public Health Act. However, results depend on the level of participation. The more people who have participated, the more accurate will be the picture of public health in the various municipalities.
- Report from Nesseby Municipality
- Report from Tana Municipality
- Report from Lyngen Municipality
- Report from Storfjord Municipality
- Report from Karasjok Municipality
- Report from Porsanger Municipality
- Report from Kautokeino Municipality
- Report from Kåfjord Municipality
- Report from Evenes Municipality, 2nd version
- Report from Skånland Municipality, 2nd version
Financial/grant information:
The study received research funding from:
- The Northern Norway Regional Research Fund
- The county councils of Nordland, Troms and Finnmark
- Northern Norway Regional Health Authority (Helse Nord)
- The Sami Parliament
- The Sámi Norwegian National Advisory Unit on Mental Health and Substance Use (SANKS)
- The Ministry of Health and Care Services
None of the above have conflicts of interest in the study.