SAMINOR 1
SAMINOR 1 was the first survey of the Population-based Study on Health and Living Conditions in Regions with Sami and Norwegian Populations.
The SAMINOR 1 Survey was conducted in 2003–2004 in collaboration between the Centre for Sami Health Research (CSHR) at the Department of Community Medicine, UiT The Arctic University of Norway and the National Health Screening Service, which was eventually incorporated into the Norwegian Institute of Public Health. SAMINOR 1 was conducted in parallel with a regional health survey (TROFINN) organised by the Norwegian Institute of Public Health.
Municipalities invited to participate
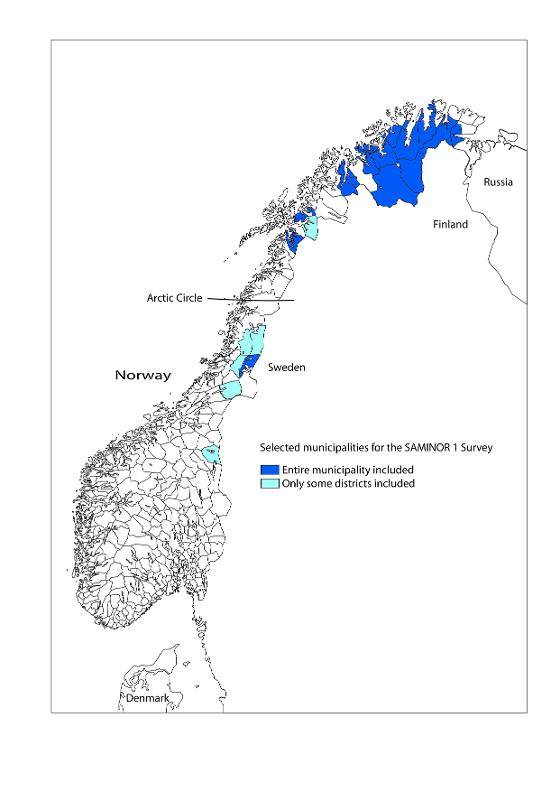
- Finnmark:
Karasjok, Kautokeino, Porsanger, Tana, Nesseby, Lebesby, Alta, Loppa and Kvalsund.
- Troms:
Kåfjord, Kvænangen, Storfjord, Lyngen, Skånland and Lavangen
- Nordland:
Tysfjord, Evenes, Hattfjelldal (kretsen Hattfjelldal), Grane (kretsen Majavatn), Narvik (the district Vassdalen)
- Nord-Trøndelag:
Røyrvik and parts of Namsskogan (Trones and Furuly) and Snåsa (Vinje)
- Sør-Trøndelag:
Parts of Røros kommune (Brekken)
Apart from Alta, all municipalities had fewer than 4000 inhabitants.
Participation
The numbers of participants referred to here are those who completed at least one questionnaire and/or underwent the clinical examination and who did not refuse consent for the use of their data in medical research.
|
|
Men |
Women |
Total |
||||||
|
Age |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
No. invited |
No. of participants |
% |
|
30 |
427 |
125 |
29.3 |
409 |
202 |
49.4 |
836 |
327 |
39.1 |
|
36-39 |
1 646 |
710 |
43.1 |
1 546 |
870 |
56.3 |
3 192 |
1 580 |
49.5 |
|
40-44 |
1 948 |
971 |
49.8 |
1 788 |
1 158 |
64.8 |
3 736 |
2 129 |
57.0 |
|
45-49 |
1 997 |
1 124 |
56.3 |
1 823 |
1 207 |
66.2 |
3 820 |
2 331 |
61.0 |
|
50-54 |
2 135 |
1 259 |
59.0 |
1 809 |
1 305 |
72.1 |
3 944 |
2 564 |
65.0 |
|
55-59 |
2 073 |
1 274 |
61.5 |
1 722 |
1 225 |
71.1 |
3 795 |
2 499 |
65.8 |
|
60-64 |
1 484 |
957 |
64.5 |
1 311 |
946 |
72.2 |
2 795 |
1 903 |
68.1 |
|
65-69 |
1 158 |
747 |
64.5 |
1 177 |
792 |
67.3 |
2 335 |
1 539 |
65.9 |
|
70-74 |
1 026 |
589 |
57.4 |
1 058 |
654 |
61,8 |
2 084 |
1 243 |
59.6 |
|
75-79 |
647 |
354 |
54.7 |
803 |
396 |
49.3 |
1 450 |
750 |
51.7 |
|
Total |
14 541 |
8 110 |
55.8 |
13 446 |
8 755 |
65.1 |
27 987 |
16 865 |
60.3 |
Implementation of the survey
When the survey began in Nesseby, a two-page questionnaire (initial questionnaire) compiled by the CSHR was sent to selected age groups, with an invitation letter and an information brochure. Those invited could choose between only the initial questionnaire or the questionnaire and the clinical examination. The completed questionnaires were returned to the Department of Community Medicine at the University of Tromsø. Those who were also interested in the clinical examination, subsequently received an invitation indicating the time and place. The invitation was sent out with a three-page questionnaire (main questionnaire) from the Norwegian Institute of Public Health (Northern Sami version of the main questionnaire). Completed main questionnaires were handed to staff at the appointment for the clinical examination. The examinations took place in two health buses parked at one or more locations in the municipality. After the clinical part of the health survey, participants were asked to complete a four-page supplementary questionnaire (Northern Sami version of the supplementary questionnaire, English translation of the supplementary questionnaire) from the CSHR and return it by post to the University of Tromsø. The study design was changed after data collection was completed in four municipalities in Finnmark: Nesseby, Tana, Karasjok and Kautokeino. The reason for this was low attendance at the clinical examination. In the remaining municipalities, all participants were invited directly to attend a clinical examination. The initial two-page questionnaire from the CSHR was added to the clinical questionnaire from the Institute of Public Health; participants therefore received a five-page questionnaire (Northern Sami version, English translation), which they handed in at the clinical examination.
Reminders
- In the first four municipalities, reminders were sent to those who had not completed the initial questionnaire, in order to increase the response rate before invitations to the clinical examination were sent out.
- In Finnmark and Troms, there was a second round, where the health buses returned to the municipalities 2-3 months later. Those who did not attend in the first round received a reminder with a new appointment in the second round.
- In the areas of Nordland, and Trøndelag, there was no second round.
- A reminder was sent to all participants who did not return the supplementary questionnaires handed out after the clinical examination.
- In the first four municipalities (Nesseby, Tana, Karasjok and Kautokeino), attendance was so low after the first round that it was decided to invite all participants to attend a clinical examination in the second round, irrespective of whether or not they had completed the initial questionnaire. This meant that many participants had a clinical examination without ever having responded to the initial questionnaire. Therefore, in 2006, those who had not responded were contacted again and data on their language and ethnicity were obtained.
For further details of SAMINOR 1, see this article:
Design 1 was used in Nesseby, Tana, Karasjok and Kautokeino. Design 2 was used in the remaining municipalities.
- Information brochure, design 1
- Information brochure, design 2
- Invitation letter, Norwegian and Northern Sami, design 1
- Initial questionnaire, Norwegian and Northern Sami, design 1
- Main questionnaire, Norwegian, design 1
- Main questionnaire, Northern Sami, design 1
- Main and initial questionnaires, Norwegian, design 2
- Main and initial questionnaires, Northern Sami, design 2
- Main and initial questionnaires, English translation, design 2
- Supplementary questionnaire, Norwegian
- Supplementary questionnaire, Northern Sami
- Supplementary questionnaire, English translation
- Consent statement, Norwegian and Northern Sami
- Reminder card, Norwegian and Northern Sami, design 1
- Clinical results card, Norwegian
For forskere:
Data controller
The director of UiT The Arctic University of Norway is the data controller.