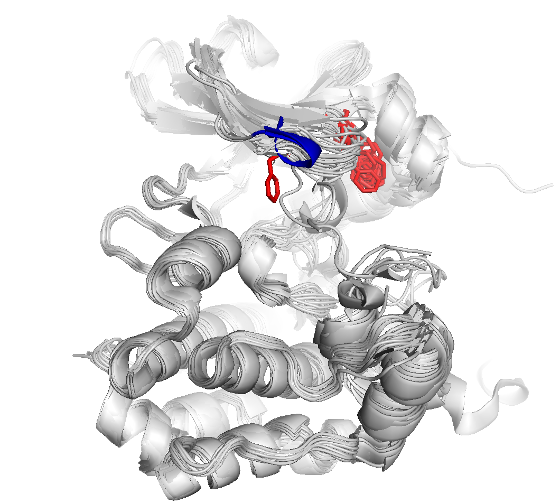
Computational studies of glycine-rich-loop conformational dynamics
Protein kinase dynamics are key determinants of catalytic activity and drug efficacy
The glycine-rich loop of protein kinases is characterized by the conserved sequence Gly-X-Gly-X-(Phe/Tyr)-Gly. The glycine residues enable flexibility, and the position of the Phe or Tyr, with its aromatic side chain, is both highly variable and important for kinase function and drug binding. A master's project would investigate the importance of flexibility using techniques and proteins selected from our ongoing research.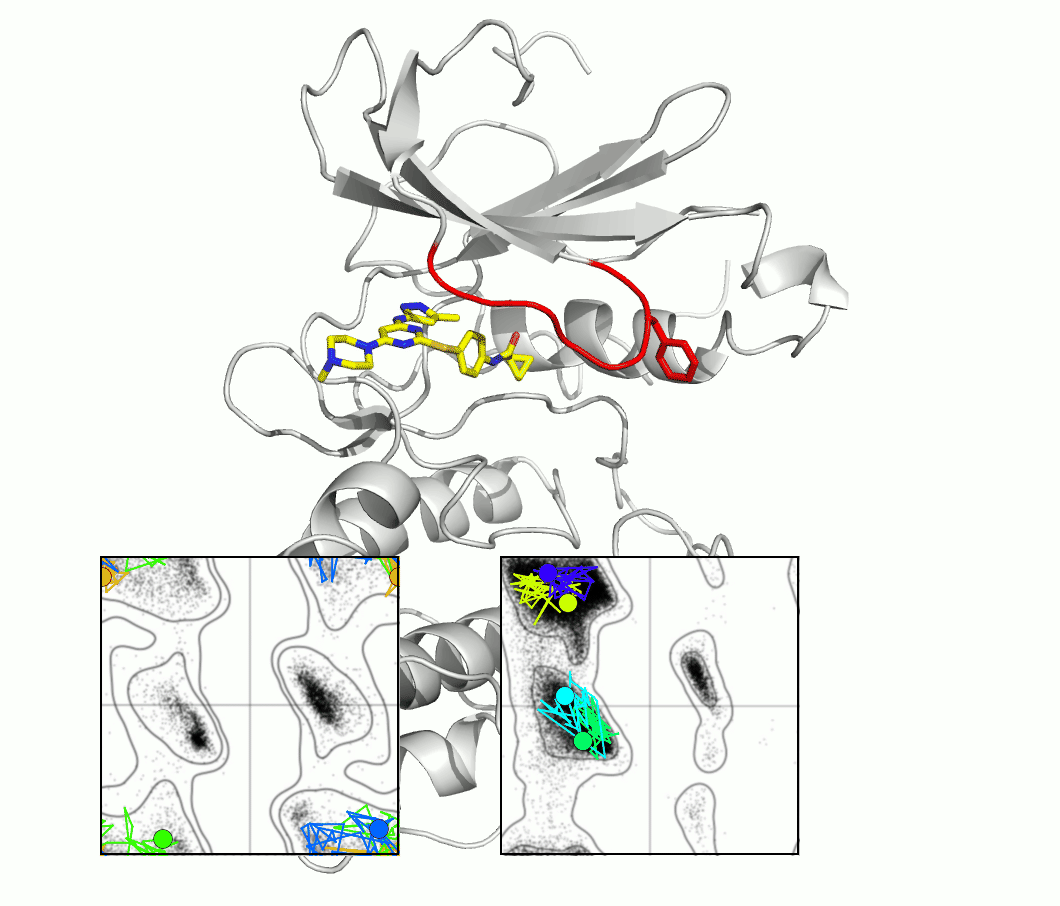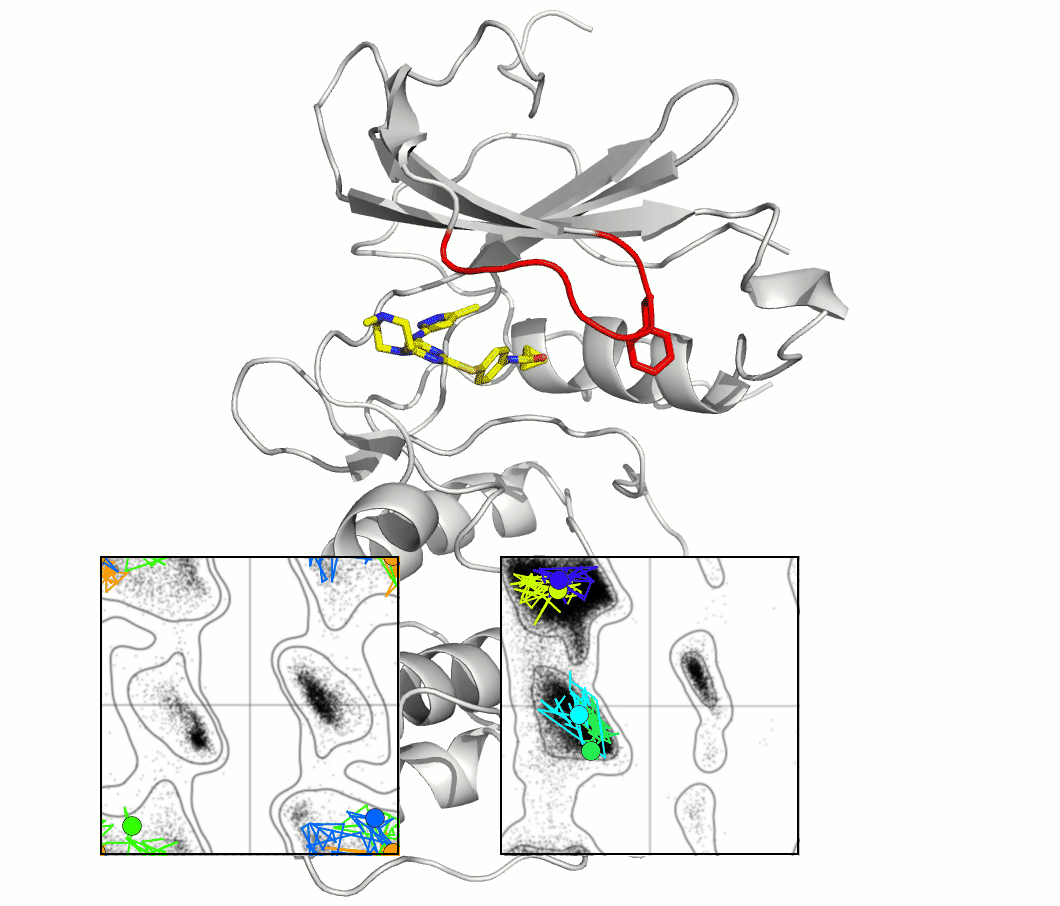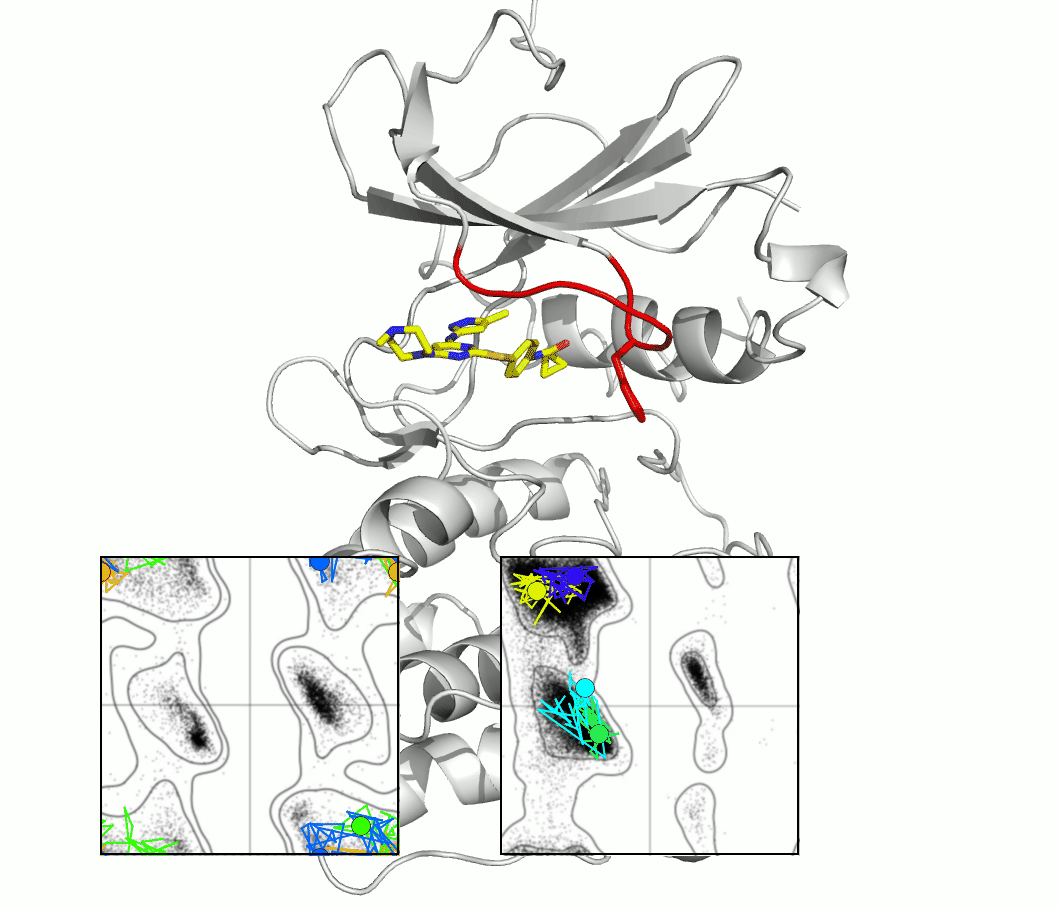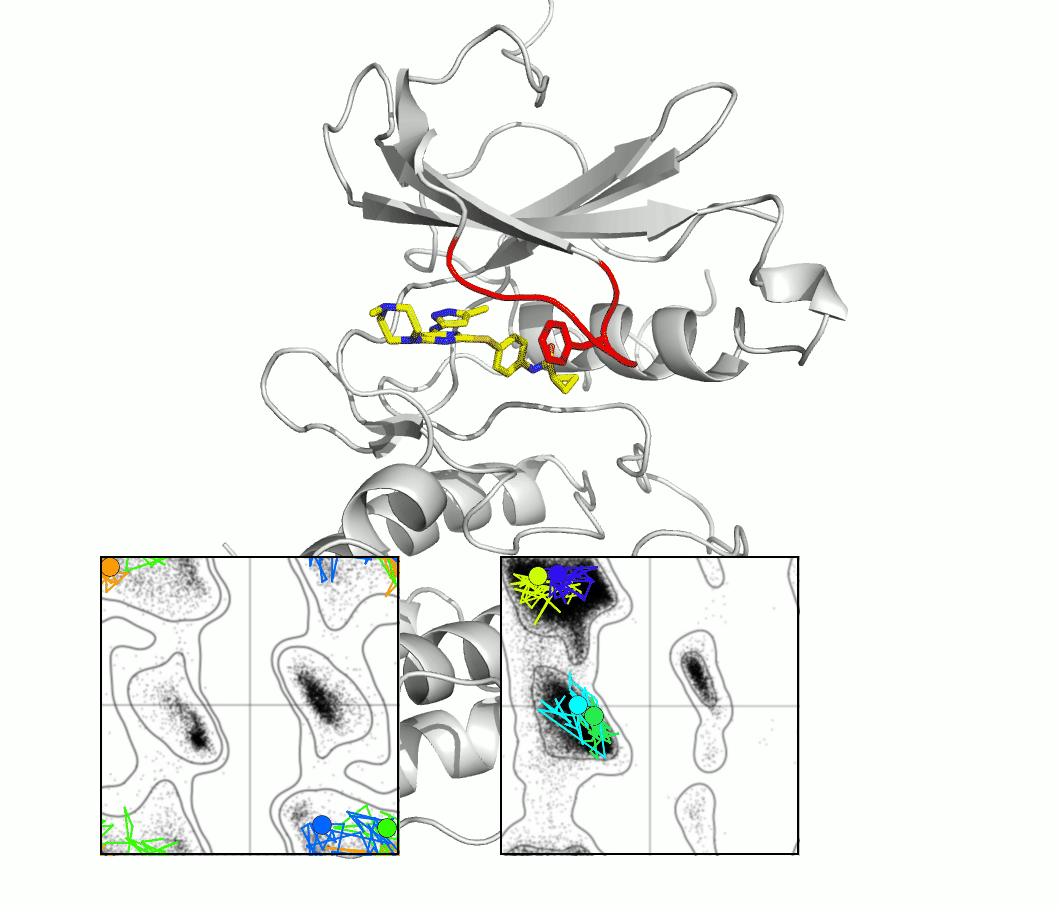
The figure shows snapshots from an MD simulation of the protein kinase Aurora, as the glycine loop undergoes a conformational rearrangement seen in crystal structures. The inset graphs show how individual amino acid main chain residues change their conformation within the energy contours derived from distributions of Ramachandran plots. One question regards the frequency of high energy conformational transitions, which may be rate limiting.