Research
Our lab has research interests spanning across the broad field of organic chemistry. Synthetic organic chemistry is our focal point, but our research also finds applications across chemistry, chemical biology and materials science.
Stable organic radicals
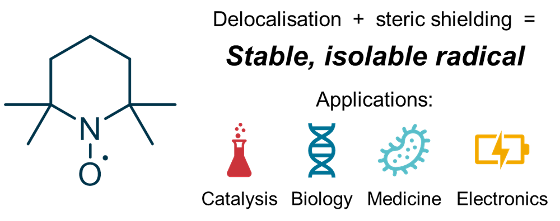
Radicals are molecules that contain an unpaired electron. While these are normally very reactive species, it is possible to stabilize them to the extent that they have practically indefinite half-lives. A typical example of a stable organic radical is 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO), which is stable to air and moisture, and is even commercially available. Apart from being fascinating in their own right, stable organic radicals such as TEMPO are used for a number of important applications, e.g. in catalysis, biomedicine and electronic materials. Our lab is researching the factors that make stable organic radicals so stable, with an aim to develop new classes of stable organic radicals with even more exciting properties and applications.
Late-stage functionalization
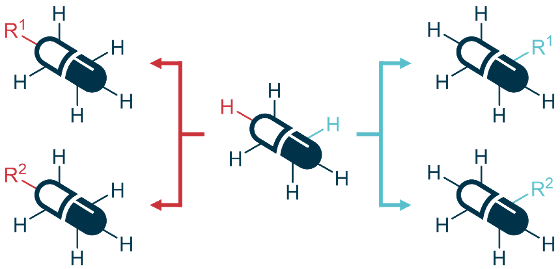
The development of new pharmaceuticals requires the preparation of a large number of analogues to elucidate and optimise the relationship between chemical structure and bioactivity. Typically, several synthetic steps are required to produce each analogue, making the drug development process laborious, slow and expensive. An alternative approach is provided by late-stage functionalization, where an already complex intermediate is modified to give access to a range of analogues, with a greatly reduced total number of synthetic steps required. Our lab is researching new methods for late-stage functionalization of pharmaceuticals, especially antibiotics, to create new analogues and derivatives. In particular, we are interested in introducing functional groups amenable to 'click' chemistry, thereby making it possible to rapidly access more complex drug derivatives.
New antibiotics and methods to counteract antibacterial resistance
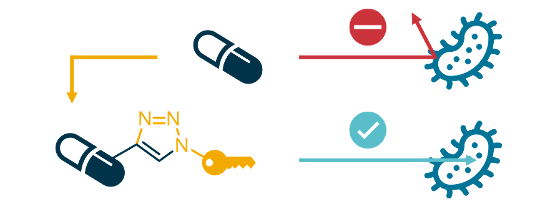
While the number of infections by bacterial strains that are resistant to treatment with existing antibiotics is rapidly increasing, the rate of development of new antibiotics has been low for the past few decades. Thus, society is approaching a 'post-antibiotic era' where common infections become untreatable once more. As antibiotics and infection control underpin large parts of modern medicine (e.g. surgery and cancer treatment), this would have dramatic consequences for humanity. One way of addressing this problem is to develop new antibiotics which are active towards resistant bacteria, and which are inherently resilient towards themselves becoming subject to resistance. Through our affiliation with the Centre for New Antibacterial Strategies (CANS) at UiT, our lab is investigating the effect of antibiotic conjugation on resistance. Conjugates are antibiotics which have been chemically linked to other molecules, thereby producing new drugs that can have higher antibiotic activity due to improved target delivery, selectivity for pathogens, or even achieve completely new mechanisms of action.
Lipid bilayers and transmembrane protein channels
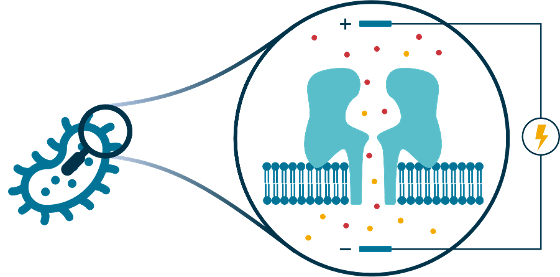
Patch clamp electrophysiology is a powerful technology for studying the properties and behaviour of synthetic planar lipid bilayers, or transmembrane protein pores inserted into such bilayers, based on the electrical conductivity across the bilayer or pore channel. The technique is highly sensitive, to the point where it can detect the formation of a single protein pore, and even observe chemical events and processes inside the pore at the single-molecule level. Our lab uses patch clamp planar lipid bilayer techniques to study membranes and transmembrane protein pores in the context of antibacterial resistance. For example, we are interested in how antibacterial compounds affect membrane integrity or influence the behaviour of protein channels that are important for cellular drug influx or efflux.